Abstract
Background Acute myeloid leukemia (AML) accounts for 15-20% of all pediatric acute leukemias and is a very heterogeneous disease, both genetically and phenotypically. Although survival has increased gradually during the last decades, relapse-rates remain around 20-30%, and children who relapse have a relatively poor outcome. Still, the exact cause of pediatric AML (pAML) remains unclear. Previously, we found an increased clonal mutation burden in leukemic blasts of a subset of pAML cases, including the ones characterized by RUNX1-RUNX1T1 translocations. Here, we study the mutation accumulation and clonal evolution of pAML to elucidate the timing of driver accumulation and mutational processes underlying leukemogenesis in children.
Methods All pAML bone marrow samples were acquired via the biobank of the Princess Máxima Center for pediatric oncology after ethical approval was obtained. We used primary template-directed amplification (PTA) to accurately analyze the complete genomes of single cells at nucleotide resolution. For PTA-mediated whole genome sequencing (WGS), we sorted single leukemic blasts, hematopoietic stem cells (HSCs), multipotent progenitor cells (MPPs) and several differentiated cell types. Subsequently, WGS data was used to study mutation accumulation and to perform phylogenetic lineage tracing of different cell types of the same patient.
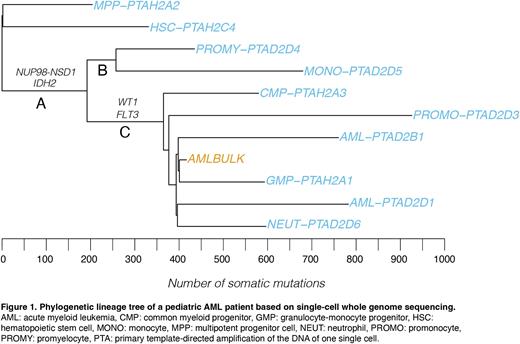
Results Thus far, we have analyzed the single-cell WGS data of 18 cells from two AML samples characterized by NUP98-NSD1 and RUNX1-RUNX1T1 fusions. We identified multiple cells that immunophenotypically resembled HSCs, MPPs and more mature cell types from different lineages, that shared the fusion gene with the AML blasts (Figure 1). Moreover, a subset of these cells shared one or more additional driver mutations with the immunophenotypically defined bulk leukemic blasts. Subsequent mutation analysis showed that cells with the oncogenic fusion had an increased somatic mutation burden compared to cells without the fusion. Accordingly, we studied mutational signatures to identify the processes underlying this mutation burden. Interestingly, besides the expected mutational signatures, we found that the increased mutation burden was mainly characterized by SBS18, a signature that is associated with damage induced by oxidative stress. This process was active throughout the leukemic lineage, whereas unrelated HSCs and MPPs of the same patients did not display this signature. This finding indicates that the process was specific for the leukemic lineage and therefore likely cell intrinsic.
Next, we used driver and passenger mutations to construct phylogenetic lineage trees and found that the accumulation of drivers occurred stepwise. We show that the oncogenic fusion was the first driving event in different patients, followed by accumulation of additional driver mutations. For example, in one patient, based on the clock-like mutation burdens in the separate branches, we could estimate that the first drivers were already present when the patient was between 7.5 to 9 years old (Figure 1, branch A), while this patient was diagnosed at 15.2 years. We have previously identified similar patterns in multiple MLL-rearranged therapy-related AML (t-AML) patients. Both in the t-AML and pAML cases, some leukemic blasts did not have any additional genetic drivers compared to the phenotypical stem and progenitor cells.
Conclusions We used single-cell WGS to identify pre-leukemic cells in bone marrow samples of pAML patients, which shared both the oncogenic rearrangement and additional driver mutations with the AML blasts. These pre-leukemic cells showed an increased mutation load, caused by distinct mutational processes. In addition, these cells were immunophenotypically and functionally stem-cell like, contributing to hematopoiesis. Our analyses show that, despite their aberrations, these cells still have differentiation potential. Interestingly, the first driver aberrations of pAML originated years before diagnosis. These data suggest that genetic driving aberrations may not be the only underlying mechanism of AML development in children. Noncoding drivers, epigenetic changes or environmental influences may play an important role in the final steps towards leukemogenesis.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal